
Acquired canaliculocele with intranasal extension in a dog
Abstract
- To describe a case of a nasolacrimal canaliculocele with intranasal extension in a dog. A 6-year-old neutered female Jack Russell Terrier was referred to the Centre Hospitalier Vétérinaire des Cordeliers for a slowly enlarging mass adjacent to the medial canthus of the right eye of 5 months duration.
Auteur :Drs. Philippe Durieux,* Stéphane Libermann,* Elise Rattez,* Marie Lagadic,† Yannick Ruel‡ and Thomas Chen§ 2015
E-mail : pdurieux@chvcordeliers.com
Mots clefs : canaliculocele, CT, dog, endoscopy, marsupialisation, nasolacrimal
Cet article a été publié dans : American College of Veterinary Ophthalmologists, Veterinary Ophthalmology, 18, 69–77
*Centre Hospitalier Vétérinaire des Cordeliers, 29 Avenue Joffre, 77100, Meaux, France; †Laboratoire Idexx Alfort, 17 Allée Jean Baptiste Preux, 94140 Alfortville, France; ‡Clinique vétérinaire Advetia, 5 Rue Dubrunfaut, 75012, Paris, France; and §Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, 80523-1601, USA
- Ophthalmic exam, ultrasound, fine needle aspiration, dacryocystorhinography and computerized tomography examinations were performed. Rhinoendoscopic treatment with marsupialisation of the endonasal canaliculocele was performed. Physical examination revealed a 3 cm diameter, subcutaneous mass beneath the medial canthus of the right eye, with a defect in the maxillary bone below.
- Dacryocystorhinography demonstrated blockage at the level of the nasolacrimal duct. Fine needle aspiration confirmed the cavitary nature of the lesion. During rhinoendoscopy, a mass containing watery fluid was found. Following marsupialisation with a large mass wall resection, the lacrimal drainage system irrigated freely in the nasal chamber. The communication of the lumen of the diverticulum with the lumen of the superior but not the inferior canaliculus was clearly demonstrated during endoscopy.
- Given negative immunohistochemical staining for smooth muscle actin and desmin, nasolacrimal dacryocele appears to be the most likely diagnosis. Histological examination confirmed that no goblet cells were present in the wall of the diverticulum, which is consistent with the canaliculus rather than the lacrimal sac. Complete resolution was observed without recurrence one year after surgery. Despite its rarity, a canaliculocele should be considered in adult dogs with nasolacrimal duct obstruction. Marsupialisation seems to be the appropriate therapy.
Acquired canaliculocele with intranasal extension in a dog
Introduction
Noninflammatory swellings ventromedial to the eye are uncommon in dogs. Possible origins have been described, including epidermoid cysts,1–3 maxillary bone epithelial
cysts,4 and other cysts, mucocele and dilatation of the periorbital glandular and duct tissues such as the zygomatic salivary gland,5 the lacrimal gland,5–7 the third eyelid lacrimal
gland,5 the lacrimal excretory system,8–10 the conjunctival epithelium, and the nasal or frontal sinus mucosa.5
Congenital conditions affecting the lacrimal drainage system include abnormalities such as atresia of the lacrimal punctum,9 ectopic lacrimal punctum and canaliculus, atresia of the lacrimal canaliculus and lacrimal sac,9 dacryops, 6,11 and canaliculops.12
Acquired nasolacrimal duct obstructions are common in adult dogs and are often associated with dacryocystitis due to foreign-body obstruction or infection.9,12–14 Less frequently, extraluminal lesions may be neoplastic,9,15,16 cystic,17–20 traumatic (maxillary and lacrimal bone fractures), 8 or inflammatory in origin19,21 when the nasal sinuses may impinge on the nasolacrimal duct. Canalicular cysts or canaliculoceles may result from the progression of congenital canalicular ectasia.9 Epiphora is frequently the major clinical sign with concurrent excoriation and conjunctivitis.9,10

Figure 1. Photograph of a 6-year-old Jack Russell Terrier neutered female dog. Note the mass around the medial canthus of the right eye of 5-month duration. No ocular discharge was observed.
Minimally invasive surgery of a lacrimal drainage system cyst with endonasal extension is poorly documented in dogs.10
In this report, we describe an unusual occurrence of a canaliculocele with intranasal extension and destruction of the adjacent maxilla. Plain radiography was used to identify anatomical abnormalities in the periorbital region. However, computerized tomography (CT) provided more precise morphological information concerning the lesion. We performed endoscopic endonasal marsupialization resulting in recovery of this dog. A definitive diagnosis was only achieved with histological categorization following the surgery. No recurrence was observed during follow-up.
Case report
Case history
A 6-year-old female neutered Jack Russell Terrier was referred to the Centre Hospitalier Vétérinaire des Cordeliers for a slowly enlarging mass around the medial canthus of the right eye of 5-month duration (Fig. 1). Intermittent serous ocular discharge had been noted, with nasal stridor and more recently episodic sneezing. The referring veterinarian had diagnosed an abscess of the root of the fourth premolar and removed the carnassial tooth and the two upper right molars 4 months earlier. Systemic therapy was used in combination with surgery and consisted of the fluoroquinolone antibiotic marbofloxacin (4 mg/kg orally once daily administered for 15 days, Marbocyl® 20 mg; Vetoquinol, Lure, France) and the nonsteroidal anti-inflammatory agent tolfenamic acid (4 mg/kg orally once daily administered for 3 days, Tolfédine® 20 mg; Vetoquinol). No improvement was noted. Aspiration of the mass, 2 months before referral, produced watery fluid and size reduction, but the mass enlarged again within a few days.
The swelling was not associated with any discomfort. There was no history of prior ocular or periocular inflammation or trauma.
Medical treatment was discontinued for 3 months.
Clinical findings
There was no evidence of systemic disease during the general physical examination. Complete blood count and routine biochemistry were within normal limits.
Physical examination revealed a 3-cm-diameter, firm, round, immobile subcutaneous mass beneath the medial canthus of the right eye, overlying the maxillary bone. Palpation of the maxillary bone revealed a defect in the bone below the area of soft tissue swelling. Upon applying digital pressure to the mass, there was no fluid reflux through the lacrimal puncta, but some movements of the eye were present. Inflammatory signs such as erythema were not found over the swelling. Palpation of regional lymph nodes revealed no abnormalities.
Applanation tonometry revealed intraocular pressures of 14 mmHg in the right eye (OD) and 13 mmHg in left eye (OS) (Tono-Pen® XL; Mentor Worldwide LLC, Santa Barbara, CA, USA). Values for the Schirmer tear test (Laboratoire TVM, Lempdes, France) were 16 and 12 mm/min OD and OS, respectively. A normal menace response and palpebral reflex were present in both eyes, and the direct and consensual pupillary light reflexes were also normal. Slit-lamp microscopy (SL-15 Portable Slit-Lamp Biomicroscope; Kowa Co. Ltd, Tokyo, Japan) and fundoscopy (Omega® 500 LED indirect ophthalmoscope; Heine Optotechnik, Herrsching, Germany and Volk Pan Retinal 22 diopter indirect ophthalmoscopy lens; Nidek S.A., Saint-Priest, France) were completed, and no abnormalities were noted. Fluorescein dye (Fluorescéine® 0.5% unidose eye drops; Laboratoire TVM) applied topically to each eye appeared in the left nares within 3 min, but did not appear in the right after 20 min.
A 24-gauge nasolacrimal metal cannula preplaced on a 5-mL syringe containing saline solution, and a drop of topical anesthetic (Tetracaine® 1% eye drops, Laboratoire TVM) was applied to the conjunctiva. The canaliculi of the right eye were cannulated, and a small volume of saline solution injected while observing the other lacrimal punctum. Abnormal resistance and relative distension of the mass was noticed during attempts to flush saline through the cannulated superior or inferior canaliculus. It was not possible to flush saline from the superior to the inferior punctae. No saline solution was detected in the right nares.
Percutaneous fine needle aspiration of the mass was performed using a syringe attached to a 22 G needle. The mass yielded a clear, colorless, watery fluid. Laboratory analysis revealed a specific gravity of 1008, a protein content of 0.9 g/L and no cells. Bacteriology on the fluid was negative for aerobic and anaerobic growth. Fungal culture was negative.
Pre-operative imaging
The dog was premedicated with an intramuscular combination of acepromazine (0.03 mg/kg, Calmivet®; Vétoquinol, Lure, France) and morphine (0.4 mg/kg, Morphine Aguettant®; Aguettant, Lyon, France) 30 min before induction.
Anesthesia was induced with alfaxalone (2 mg/kg intravenously, Alfaxan®; Vetoquinol) to effect for tracheal intubation and isoflurane and oxygen maintenance (Vetflurane®; Virbac, Carros, France).
Evaluation of the swollen area by B-mode ultrasonography revealed an anechoic cavitary structure, lined by a thin wall. Posterior acoustic enhancement was present (My-Lab®One; 15–22 MHz linear array transducer; Esaote France, Saint-Germain-en-Laye, France).
Dorsoventral and lateral radiographs of the skull were obtained and revealed a 10-mm-wide lucent area dorsal to PM3 and PM4 suggesting lysis of the maxillary bone and underlying turbinates (High Frequency X-ray Generator Sedecal compact 320®; Sedecal, Madrid, Spain) The bony limits of the lytic area were smooth and well delineated, consistent either with pressure lysis due to a slow-growing mass or a very aggressive lytic lesion.
Dacryocystorhinography was performed on the right side by catheterizing the right superior lacrimal duct with a 24-gauge nasolacrimal metal cannula and injecting 4 mL of a 1:1 dilution of iohexol (Omnipaque 300®, GE Healthcare SA, Velizy, France, 300 mg iodine/mL) and isotonic saline solution. Dorsoventral and lateral radiographs were obtained. Contrast medium accumulation was visible in the area of the right maxillary recess; filling of the proximal part of the nasolacrimal duct could not be achieved (Fig. 2).
For further assessment, the dog underwent computerized tomography (CT) that extended from the lower aspect of the orbits through the nasal cavity. The computerized tomography studies were carried out using a 16-row multislice CT scanner (Philips Brilliance Mx 8000 IDT 16 CT Scanner, Philips Medical Systems, Hamburg, Germany). Two-millimeter computerized tomographic images (100 mA, 120 kV) were acquired, with both bone and soft tissue algorithms, from the orbital region to the rostral part of the nasal cavity to cover the length of the lacrimal duct. A 10-mm-wide bone defect was observed in the dorsolateral part of the right maxillary bone, rostral to the orbital cavity, at the level of the P3 to M1 tooth roots (see image). Its borders were smooth, and no proliferation could be seen. A soft tissue density, 17 mm wide and 20 mm long, occupied the ventrolateral part of the nasal cavity at that level, slightly protruding through the bone defect and severely reducing the nasal cavity diameter. After IV iodinated contrast injection (ioxitalamate 700 mg/kg IV, Omnipaque 350®, Guerbet, Roissy CDG, France), a thin rim enhancement was observed around this mass, suggesting the presence of a thin wall (Fig. 3).
Three-dimensional reconstruction of CT image clearly demonstrated a bone defect of the maxillary, lacrimal, and frontal bones (Fig. 4).
The diagnostic imaging results were consistent with lacrimal duct dilatation, suggesting a lacrimal obstruction either due to endoluminal filling or parietal stenosis, because no periductal mass could be demonstrated. Given the extent of the mass, its effect on breathing and its cavitary nature, an endoscopic treatment with incision, decompression, and marsupialization of the endonasal lesion was recommended and scheduled.
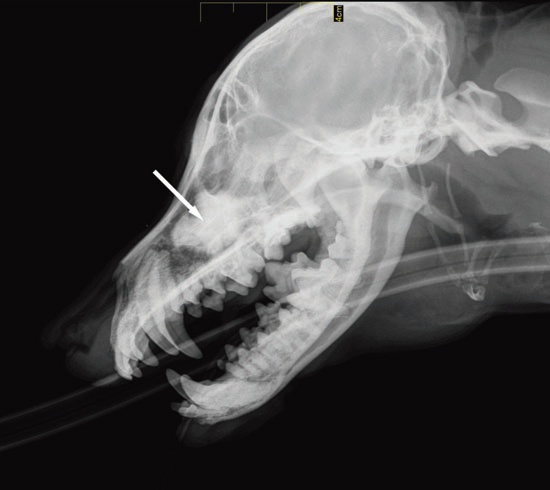
Figure 2 a
Lateral view
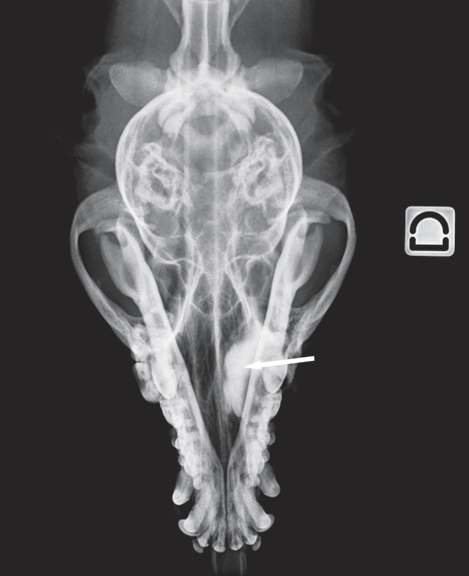
Figure 2 b
Dorsoventral view
Fig. 2 a,b : Views of the dacryocystorhinogram of the right nasolacrimal system in a 6-yearold Jack Russell Terrier neutered female dog. Contrast material accumulated in a cystic cavity (arrows) within the right maxillary sinus but did not extend into the nasolacrimal duct distal to the cystic cavity.
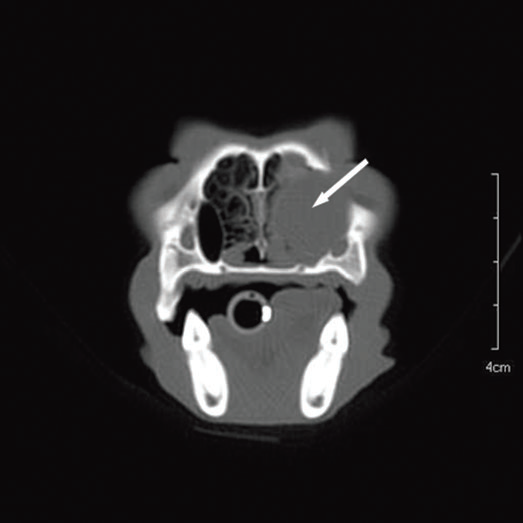
Figure 3 a
Transverse view
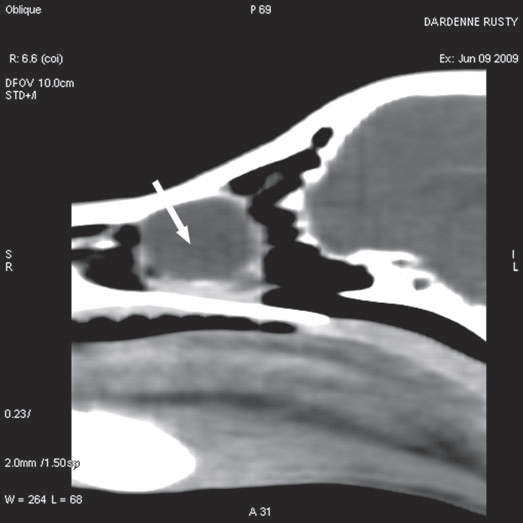
Figure 3 b
Sagittal view
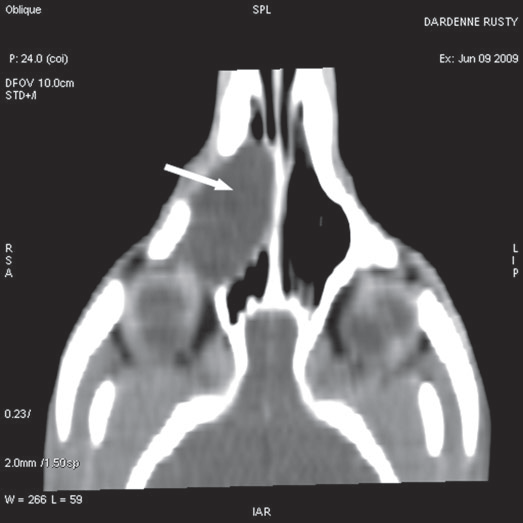
Figure 3 c
Coronal view
Fig. 3 a,b,c : computerized tomography (CT) imaging at the orbit and pterygopalatine fossa level. A well-circumscribed cystic structure (arrows) can be identified extending into the orbit and nasal cavity.
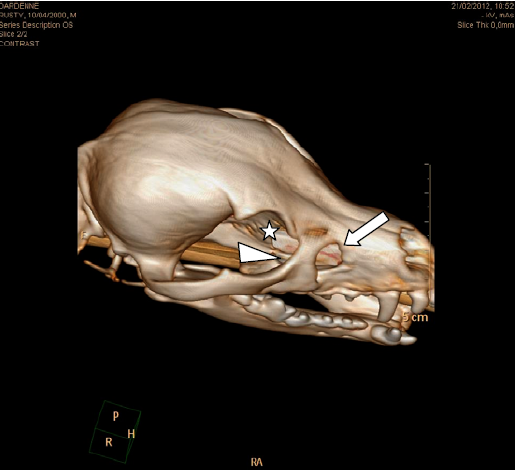
Figure 4
Three-dimensional volume rendering reconstruction of the computerized tomography (CT) imaging. It demonstrated the defect in the right maxillary (arrow), lacrimal (arrowhead), and frontal (asterisk) bones more clearly.
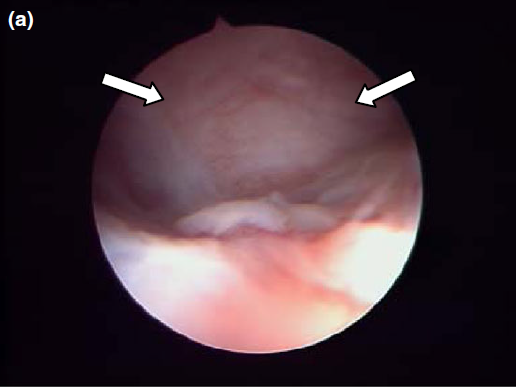
Figure 5 a
Endoscopic endonasal view of the right dorsal meatus, showing the cyst filling the meatus before marsupialization (arrows).
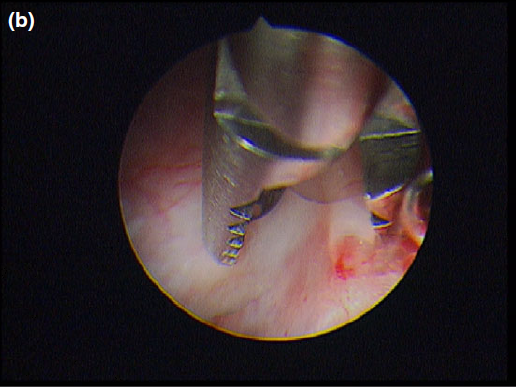
Figure 5 b
The cyst wall was opened using a straight Blakesley forceps.
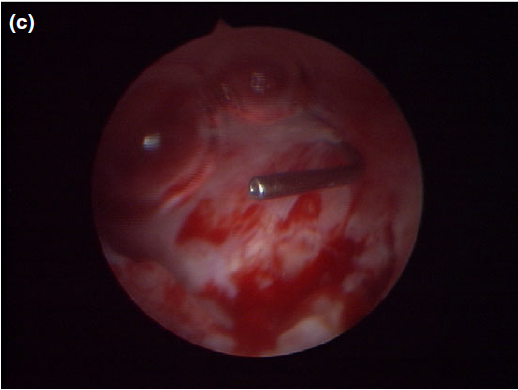
Figure 5 c
The appearance of the dorsal meatus after marsupialization and excision of the canaliculocele, with the nasolacrimal duct probe still in place.
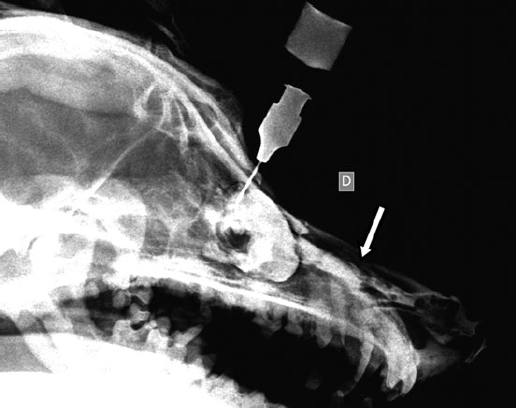
Figure 6
One year postoperatively, the contrast medium flowed freely through the nasal cavity (arrow).
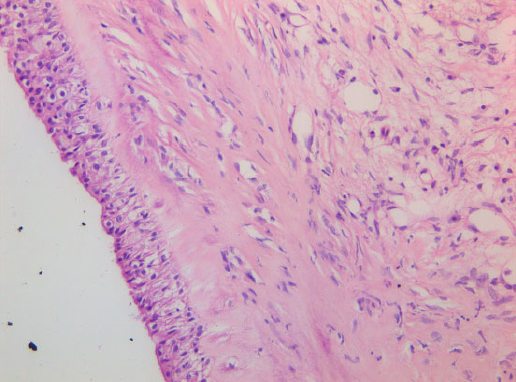
Figure 7
Cyst wall consisting of multilayered cuboidal cells with underlying fibrous tissue. 409 objective, HE stain.
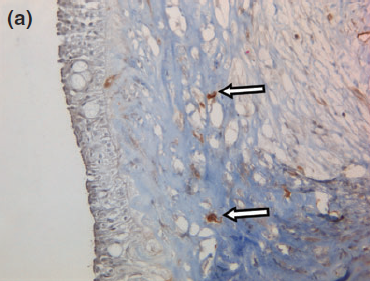
Figure 8 a
Representative photographs of the negative immunohistochemical staining for (a) smooth muscle actin and (b) desmin confirmed the absence of myoepithelial cells under the cyst wall.
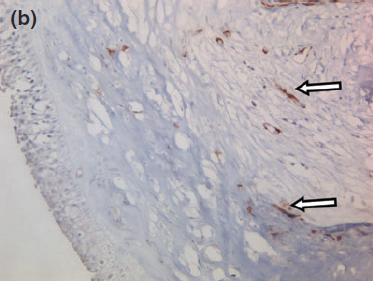
Figure 8 b
The blood vessels are surrounded by numerous myoepithelial cells, serving as a positive control (arrows). 409 objective, counterstained with hematoxylin and eosin.
Surgery
An anterograde approach was performed via the right nasal chamber. A rigid endoscope (Karl Storz Endoscopie France SAS, Guyancourt, France) with a diameter of 2.7 mm with a 30° angle and a length of 19 cm was used, coupled to a three-CCD camera (Tricam®; Karl Storz Endoscopie France SAS). This imaging system uses three separate charge-coupled devices (CCD), each one taking a separate measurement of the primary colors, red, green, or blue light.
The endoscope was inserted inside a 4-mm-diameter sheath with two Luer-lock taps for irrigating with fluids and aspiration of secretions, with a large (1.65 mm) instrument channel for flexible biopsy and grasping forceps. Continuous irrigation was provided by an arthroscopic irrigation set (Arthropump® plus; Karl Storz Endoscopie France SAS). This irrigation system combines fluid supply and suction in one unit and thus provides a consistently clear field of view.
Nasal endoscopy showed a mass in the dorsal nasal meatus. To prevent any secondary scarring, all the visible rostral cavitary mass wall was resected, thus establishing drainage. Opening of the mass was performed with flexible endoscopic grasping forceps (Type 67761 T®; Karl Storz Endoscopie France SAS) introduced through the operating channel of the optical sheath. The opening was then enlarged with larger rigid grasping forceps (Blakesley® Nasal Forceps type 456500; Karl Storz Endoscopie France SAS) introduced parallel to the rhinoscope under visual guidance. Only the cavitary mass wall surface in contact with and firmly attached to the adjacent osseous surface was left intact (Fig. 5).
Following marsupialization, the lacrimal drainage system was irrigated via the nasal chamber. No stent was placed. The superior canaliculus was cannulated, and the 24-gauge metal probe was easily visualized in the cavity of the mass (Fig. 5). The inferior canaliculus was also cannulated. The nasolacrimal probe was not visible within the cavity, but movements of the probe produced visible movements under the mass wall.
Postoperatively, the dog was treated with oral cephalexin antibiotic (15 mg/kg every 12 h, Rilexine®; Virbac) and the nonsteroidal anti-inflammatory drug carprofen (4.4 mg/kg once daily, Rimadyl®; Pfizer Santé animale, Paris, France) for 2 weeks.
One year postoperatively, the animal remains asymptomatic with no recurrence of the cyst found after radiographic examination and a dacryocystorhinogram as previously described. The contrast medium flowed freely through the nasal cavity (Fig. 6).
Histology
Histology confirmed a cystic lesion with a lining epithelium composed mostly of multilayered or pseudostratified cuboidal cells with underlying fibrosis (Fig. 7). No goblet cells or cilia could be detected. Calcification of the cyst wall, probably secondary to chronic low-grade inflammation, was noted and has been reported elsewhere.13 The histological diagnosis was a non-neoplastic cyst. Immunohistological staining for smooth muscle actin and desmin were performed and were both negative (Fig. 8).
Discussion
This dog was presented with a slowly enlarging mass around the medial canthus of the right eye of 5 months’ duration. Ultrasonography was useful in identifying the mass as a cyst.
The ultrasound definition of a simple cyst is a round or oval, circumscribed, anechoic mass with a thin or imperceptible wall. Posterior acoustic enhancement should also be present.22
Dacryocystorhinography revealed the presence of a communication between the cyst-like mass and the lacrimal drainage system and allowed situating the obstruction at the level of the canaliculi. A cyst by definition refers to a closed sac that has a distinct membrane and that contains air, fluid, or semi-solid matter. In our case, there was a communication with the lacrimal excretory system, which is more consistent with a diverticulum.
Featherstone et al.4 suggest performing contrast dacryocystorhinography via a retrograde approach to better define the obstructed zone. In our case, this method was not used. CT imaging showed no dacryocystitis, no trauma, and no mass. Postinflammatory stenosis of the nasolacrimal duct remains the most likely cause of the proximal obstruction of the nasolacrimal duct.
Given the negative immunohistochemical staining for smooth muscle actin and desmin, a nasolacrimal lesion appears to be the most likely diagnosis. The histological absence of myoepithelial cells in the lining rules out a dacryops, which is a cyst of lacrimal glandular ductal tissue origin as normal lacrimal glandular tissue is strongly positive for smooth muscle actin and desmin.11,23
Distal obstruction of the lacrimal outflow generally presents with dacryocystitis and epiphora.10,12,13 None of these were present in our case. This causes accumulation of fluid in the drainage system. White et al.10 have observed the same phenomenon in a 1-year-old Dachshund that also presented with a cystic nasolacrimal obstruction with endonasal cystic extension.
To produce a distension of the lacrimal excretory system, a second obstruction just anterior to the cyst is necessary.24 White et al.10 concluded that the absence of epiphora could suggest that dogs, like people, have an active transport of the tears along the nasolacrimal duct against hydrostatic pressure and that the excretory lacrimal system is able to prevent reflux of secretions by the presence of a fold of mucosa at the level of the lacrimal sac, like the Rosenmuller valve in humans.12,20,25 To our knowledge, such a structure has not been described in dogs.26
According to Cavazza et al.,24 a second possible cause of proximal obstruction is functional and caused by distension of the sac that compresses the canal system causing a trapdoor-type blockage. The tear flow is only possible in one direction, and it may explain the pressure cystic dilatation of the nasolacrimal sac.
The entity of dacryocele with endonasal cyst was recognized as early as the 19th century in human pediatric patients and has since been given many names, all alluding to different aspects of this unique pathologic condition. Among them, are lacrimal mucocele, nasolacrimal mucocele, lacrimal sac mucocele, dacryocystocele, dacryocele, lacrimal sac cyst, and amniotocele.27,28 Dacryocystocele is the more accurate term as it is anatomically accurate and makes no reference to the source of the fluid in the lacrimal sac.
In people, dacryocystocele is encountered almost exclusively in children and is believed to occur as a result of an obstruction distal to the valve of Hassner and an obstruction proximal to the valve of Rosenmuller.24,25,28–32 The valve of Hassner is a fold of mucous membrane at the lower end of the nasolacrimal duct which prevents air from being blown back from the nose into the lacrimal sac. It can remain imperforate and cause obstruction of the tear flow. The incidence of neonatal dacryocele with endonasal cyst is 0.08% in healthy neonates.27,30,33–36 Dacryocystocele rarely occurs in adults in an acquired form.17,21 Adult acquired dacryocystocele can be idiopathic 20 or a complication of chronic dacryocystitis, trauma, or neoplasia.9,14–16,37 In rare cases, proximal and distal blockages of the canaliculus might lead to distension, forming a canaliculocele.20
Although these dacryoceles or these canaliculoceles may be congenital, inflammatory, or traumatic in origin, most authors believe they arise from diverticula of the lacrimal sac or from the canaliculus, with the cyst forming when the communication to the nasolacrimal excretory system closes.4,8,38–40
In our dog, it was possible to cannulate the superior lacrimal canaliculus, and a communication from the lumen of the cyst to the lumen of the superior canaliculus was clearly demonstrated during surgery. Communication of the inferior canaliculus with the lumen of the cyst was not observed.
The presence of a diverticulum with distension secondary to the proximal obstruction of the nasolacrimal duct and the accumulation of fluid in the drainage system might have been possible. The diverticulum compresses the canal system causing a trapdoor-type blockage and allows the endonasal cystic development. A congenital predisposition due to the presence of a diverticulum seems to be possible in our adult dog although it is difficult to confirm with certainty that the lesion was acquired, as in humans, diverticula are often asymptomatic.41
Histological examination confirmed that no goblet cells were present in the walls of the cyst, which is consistent with tissue originating from the canaliculus rather than from the lacrimal sac nor the nasolacrimal duct.26 The cyst could be consistent with a canaliculocele originating from the superior canaliculus.
In humans, reported cases of canaliculocele are very rare and have involved the inferior canaliculus,20,38 the common canaliculus, that is, the common part of the two canaliculi before the lacrimal sac20 and the upper canaliculus.41 The common canaliculus is not present in dogs.26 Cysts have been described near the inferior canaliculus but without communicating with it.8,12 They are usually associated with ectopic lacrimal ducts.6,22
White also noticed in his dog, as in ours, a communication with the upper canaliculus10. He observed a distension of the mass during attempts to flush saline solution through the cannulated superior canaliculus. However, he could not confirm by nasal endoscopy a direct communication of the lumen of the superior canaliculus with the lumen of the cyst. His histological examination identified chronic inflammatory tissue. He was not able to localize the level of the cystic dilatation. Nasal endoscopy proved to be a very useful aid, and the use of modern endoscopic video equipment with a fluid management system gives a clear view of the working field, which was not the case for White.
Tomographic imaging studies, used in our case, avoid superimposition, providing better identification of the structures concerned and the extent of the lesions in comparison with radiographic studies. CT imaging provides a thorough examination of bony structures with good spatial resolution, visualizing very discrete lytic lesions. However, its soft tissue contrast is poorer than MRI studies, even with the use of iodinated contrast media, given either intravenously or intracavitary, which is usually of help in delineating lesions or anatomical landmarks.
In a recent study on the normal canine nasolacrimal drainage system by computerized tomographic dacryocystography, the detection of iodinated contrast media in the superior canaliculus was found to be difficult, but possible. 42 In our case, considerable bone remodeling caused excessive pressure by the cyst on the canaliculus preventing good filling, and the anatomical changes would probably have made this examination difficult to interpret due to the small size of these structures. Because of the uncertainty of obtaining additional, accurate information, it was decided not to conduct this examination in favor of endonasal endoscopy.
Investigation of medial canthal tumefaction may include MRI studies that can depict orbital structures,43 but MRI of the very thin lacrimal duct structures has not been described in the dog yet. However, high-field 3D MRI may provide good evaluation of these structures because T2-weighted images would show fluid content, and fat suppression sequences would allow suppression of the strong signal from the large amount of orbital fat. T1 and T1 postcontrast weighted images would delineate bones and lesions showing contrast enhancement.
Three-dimensional reconstruction of CT images clearly demonstrated a bone defect of the maxillary, lacrimal, and frontal bones. The degree of enlargement of the cystic lesion resulted in atrophy of the surrounding bone. Previous studies state that the pressure exerted as a result of the enlargement of the cyst would be probably be sufficient to cause destruction of this bony border.3,44 Some authors have suggested the possibility of the existence of osteolytic mediators produced by fibroblasts from the dacryocele lining, such as PGE2 and collagenases, which are probably the main factors responsible for bony erosion during the pressure-induced erosive phase.45
The cystic dilatation occupies approximately two-thirds of the nasal cavity. In the presence of respiratory difficulties and to avoid complications such as infection (dacryocystitis, cellulitis), surgical intervention is recommended. 19,21,25,28,31,33,46–49
Three invasive surgical therapies are typically described in veterinary medicine: conjunctivorhinostomy,10,50 conjunctivobuccostomy, 10,50 or an intra-osseous approach to perform extirpation of the cystic structure and creation of a communicating hole between the maxillary recess and the nasal cavity.13,50
In human medicine, if the lacrimal dacryocystocele is associated with endonasal cyst expansion and symptoms of airway obstruction, early surgical intervention is mandatory. Historically, in the pre-endoscopic era, the minimal procedure involved probing of the lacrimal duct in an attempt to achieve patency of the distal obstruction. If the conservative approach failed, the surgical procedure of choice was external dacryocystorhinostomy that in effect created an alternative pathway for drainage to the obstructed duct into the middle nasal meatus.27 Nasal endoscopy and cyst marsupialization to open the nasolacrimal system is today the recommended procedure as probing alone may fail to perforate the intranasal cyst or the initial opening may close. This allows the satisfactory drainage of lacrimal secretions from the proximal nasolacrimal duct directly into the nasal chamber and obviates the need for temporary cannulation.28,37,42,43,51–53
In light of the excellent results in humans, we chose to adopt the same noninvasive procedure for our patient. This technique is seldom reported in veterinary medicine, and White et al. have described only one surgical case to the knowledge of the authors.10,12
Endoscopic equipment has changed dramatically since his description of the surgical technique. A small-diameter video endoscope, an irrigation suction system giving perfect visualization, and the presence of a large instrument channel make the surgery simple and accurate.
Thanks to clear viewing of the surgical site, a large, permanent fistula is easily created in the medial wall of the intranasal component of the canaliculocele and the nasolacrimal system patency is well visualized by showing the lacrimal probe inside the canaliculus.
No recurrence of the intranasal cyst was noticed 1 year postoperatively, and the dog remains asymptomatic.
We conclude that a nasolacrimal drainage system canaliculocele should be among the differential diagnoses of a medial canthal mass in adult dogs, although it is rare. The use of nasal endoscopy is a great help in the diagnosis and treatment of dacryocele with nasal extension. As in human pediatric patients, marsupialization seems to be the appropriate therapy.
References
|